Hanson Wade Group have taken the decision to cancel this meeting. Please do accept our apologies for any inconvenience or disappointment this will cause.
Join 60+ senior industry leaders at the premier HPAPI: Highly Potent Drug Manufacturing Summit in Boston this August.
For 14 years, we’ve united some of the most knowledgeable experts across pharma, biotech, CDMOs, generics, and regulatory bodies who are all keen to shape the future of high potency drugs.
In pursuit of CMC excellence in oncology and other therapeutic areas requiring high potency compounds, we’re taking on a strategic approach this year to tackle the challenges that surround manufacturing safety, regulatory compliance, and innovation in drug development and delivery to seek incredible outcomes.
Delve into GMP-compliant scale-up strategies, innovative containment solutions, green chemistry, and collaborative process development — all tailored for handling OEB4+ cytotoxic compounds. Free* for qualified drug developers & academics:
Key Sessions You Can’t Miss:
With a 54% Big Pharma representation, learn from 15+ industry experts sharing 10+ real-world case studies to:
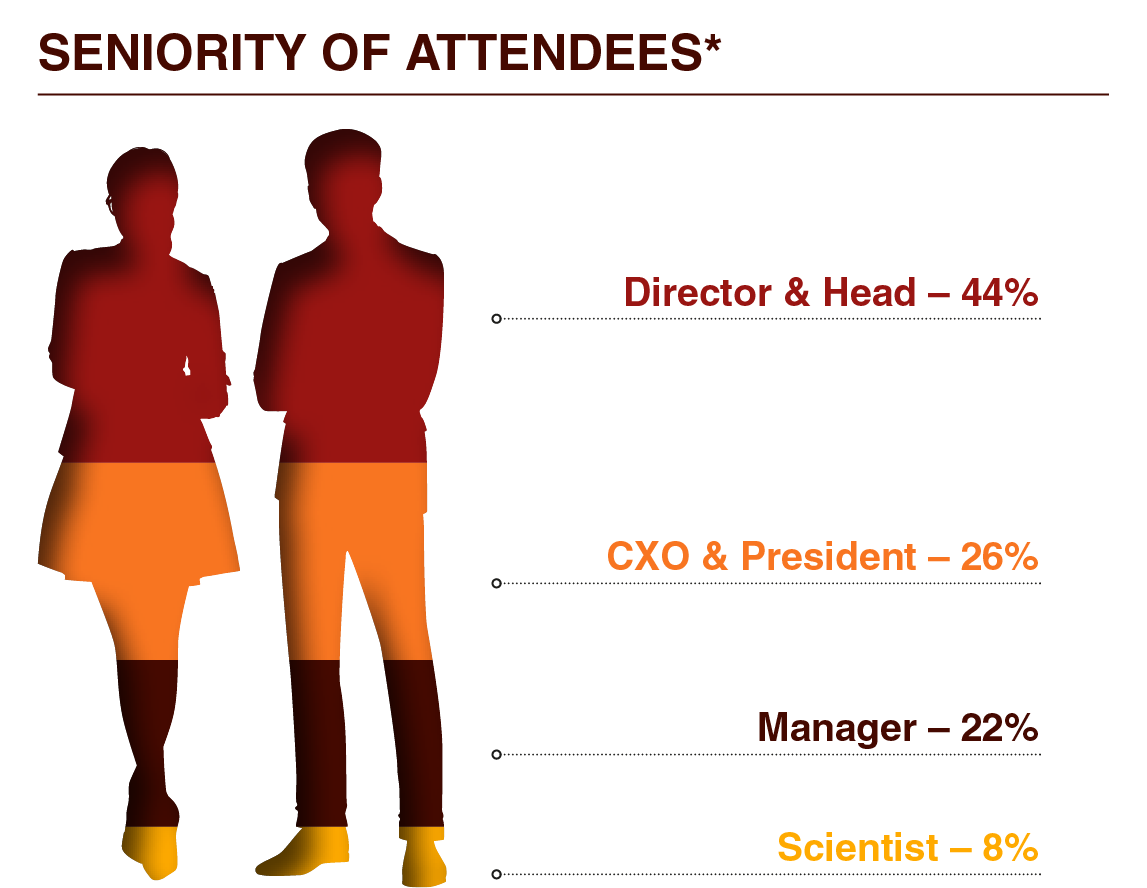
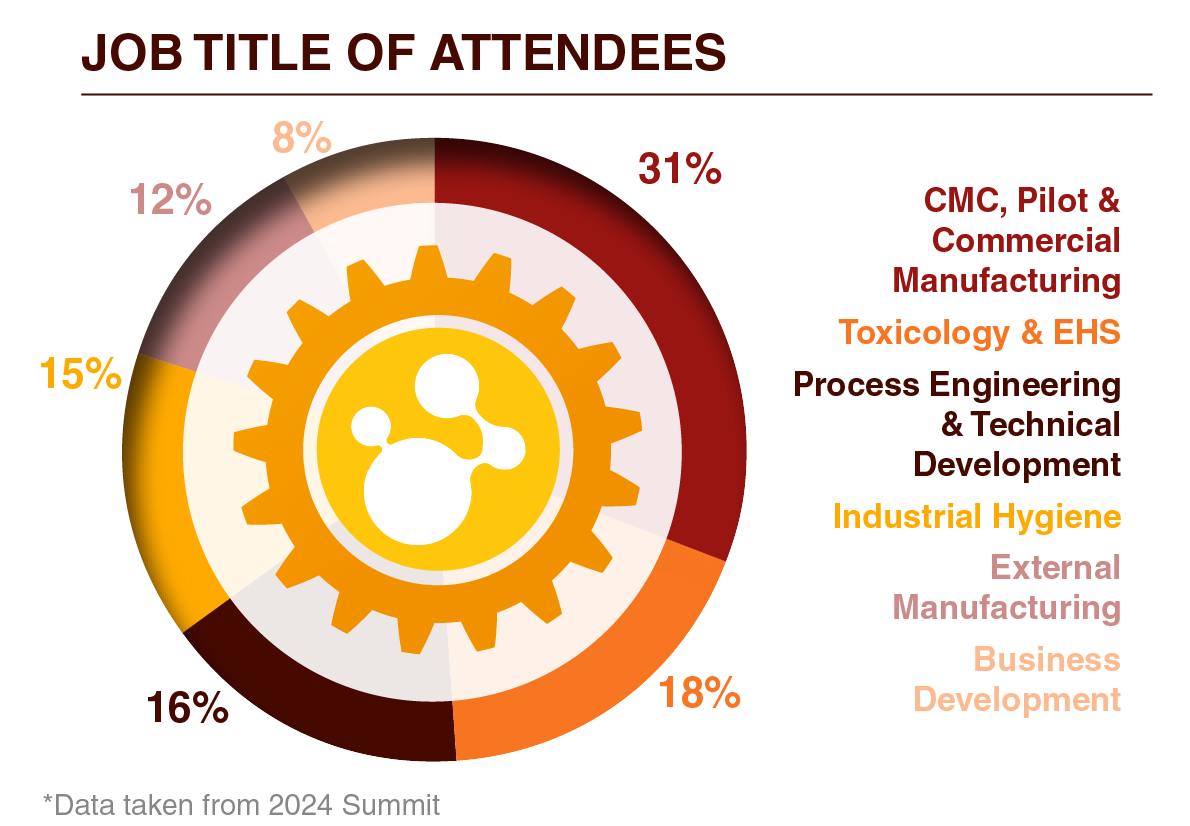
Who Will You Meet?
Our long-awaited, reputable forum is your golden ticket to join and network with 60+ Vice Presidents, Directors, and Heads of...
-
-
- CMC,
- Tech Operations,
- Process Chemistry,
- Drug Substance and Product,
- EHS,
- and Industrial Hygiene
-
...all under one roof – with the shared interest of integrating process development, green chemistry, and equipment selection to achieve GMP compliance for high potency compounds.